 |
Columbus OH (SPX) Jul 14, 2004 Ohio State University engineers and their colleagues have successfully automated a particular medical test on a compact disc (CD) for the first time - and in a fraction of the normal time required using conventional equipment. The ELISA biochemical test - one of the most widely used clinical, food safety, and environmental tests - normally takes hours or even days to perform manually. Using a specially designed CD, engineers performed the test automatically, and in only one hour. The patent-pending technology involves mixing chemicals inside tiny wells carved into the CD surface. The spinning of the CD activates the tests. In a recent issue of the journal Analytical Chemistry, the engineers report that the CD successfully detected a sample of rat antibody - a standard laboratory test - using only one-tenth the usual amount of chemicals. This first demonstration paves the way for CDs to be used to quickly detect food-borne pathogens and toxins, said L. James Lee, professor of chemical and biomolecular engineering at Ohio State. The same technology could one day test for human maladies such as cancer and HIV, using a very small cell sample or a single drop of blood. Lee estimated that the first commercial application of the concept is at least two years away. "This study shows that the technology is very promising, but there are challenges to overcome," he said. "We have been working on designing special valves and other features inside the CD, and better techniques for controlling the chemical reactions." "When we work on the micro-scale, we can perform tests faster and using less material, but the test also becomes very sensitive," he explained. As chemicals flow through the narrow channels and reservoirs carved in the CD, interactions between individual molecules become very important, and these can affect the test results. ELISA, short for enzyme linked immunosorbent assay, is normally conducted in much larger reservoirs inside a microtiter plate - a palm-sized plastic grid that resembles an ice cube tray. Microtiter plates are standard equipment in chemical laboratories, and ELISA testing is a $10-billion-per-year industry. It is the most common test for HIV. Still, the test is tedious and labor-intensive, in part because of the difficulty in mixing chemicals thoroughly enough to get consistent results. "Everyone working in the life sciences labs would fall in love with this revolutionary CD system for ELISA because it's easier, faster and cheaper to use," said Shang-Tian Yang, professor of chemical and biomolecular engineering at Ohio State and collaborator on the project. Yang and Lee are founding a company to commercialize the CD technology. Until then, product development is being handled by Bioprocessing Innovative Company, Inc., a company in which Yang is a part owner. Lee and Yang are working with Marc Madou, formerly of Ohio State and now a professor of mechanical engineering at the University of California at Irvine. Ohio State graduate student Shengnian Wang and former graduate students Siyi Lai and Jun Luo also participated in the project. While other researchers have successfully conducted ELISA testing in micro-arrays such as on a computer chip, these tests still had to be conducted manually, step-by-step. The Ohio State CD performs all the steps necessary for ELISA automatically. The special CD looks like any other on the shiny side that stores data; that is the side that is read by a laser in a computer or CD player. But the side that normally carries the disk label instead carries the tiny testing channels arranged like the spokes of a wheel. As the CD spins, centrifugal force pushes liquid samples from the inner channels out to the edge, where it mixes with tiny pools of chemicals for testing. Valves control which chemicals mix, and when. Once developed, the CD could do something else that has never been done before: merge medical information and diagnostic equipment in one platform. A patient's records and test samples could be stored on one disc. The technology will have to jump several hurdles, including evaluation by the Food and Drug Administration, before that can happen, Lee said. He sees water quality testing and food testing as two applications that could happen in the shorter term. For now, the engineers will work on optimizing the channel design for different kinds of tests. "Fortunately, once we have found a robust design, we'll only need to fine tune for each different assay, instead of inventing a totally new game again and again," Yang said. This research was funded in part by the National Science Foundation, and by Ohio Governor Bob Taft's Third Frontier program, which was designed to develop Ohio as a center for high-tech industry and create jobs across the state. Community Email This Article Comment On This Article Related Links Ohio State University SpaceDaily Search SpaceDaily Subscribe To SpaceDaily Express Space Medicine Technology and Systems
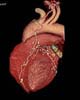 Cleveland OH (SPX) Nov 02, 2004
Cleveland OH (SPX) Nov 02, 2004With NASA looking toward extending missions to further explore space, the NASA Glenn Research Center has partnered with MetroHealth Medical Center in Cleveland to develop a method of measuring whether astronauts are more susceptible to serious cardiac episodes the longer they are in space. |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2006 - SpaceDaily.AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA PortalReports are copyright European Space Agency. All NASA sourced material is public domain. Additionalcopyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |